Can we reuse explanted devices?

Raja Selvaraj, JIPMER
Introduction
Reuse of CIED - Real scenarios
- 72 yr old physician, CRT-D for heart failure, wide QRS and VT, pocket infection
- Local device dealer - shelf expired pacemaker
- 78 yr old, CHB, CRF, Dual chamber pacemaker implanted, died in hospital
Reuse of CIED
- Reuse of explanted device in same patient
- Use of shelf expired devices
- Reuse in explanted device in other patients
Issues
- Concern about infection
- Concern about malfunction
- Reduced longevity
- Patient reluctance
- Regulatory issues
Infection
- Adequate cleaning and sterilisation
- No residual biological material
- Pyrogenic and other effects from residual chemicals
- More of an issue with hollow catheters
Malfunction
- Damage from wear and tear
- Damage during explant / cleaning
Longevity
- Lower than a new device, but generally acceptable
- Many infections occur early after implant
- Many premature deaths in patients with ICD / CRT
Patient perception
- Concern about "reused" device
- Analogy of used cars
- Analogy of organ transplant
- Scientific evidence
Regulatory issues
- Labeled by manufacturer as single use device
- Less stringent requirements
- Less liability
- The solution - "Remanufacturing"
- Validated reprocessing
Reuse is green too !
- Enviromentally good solution
Evidence
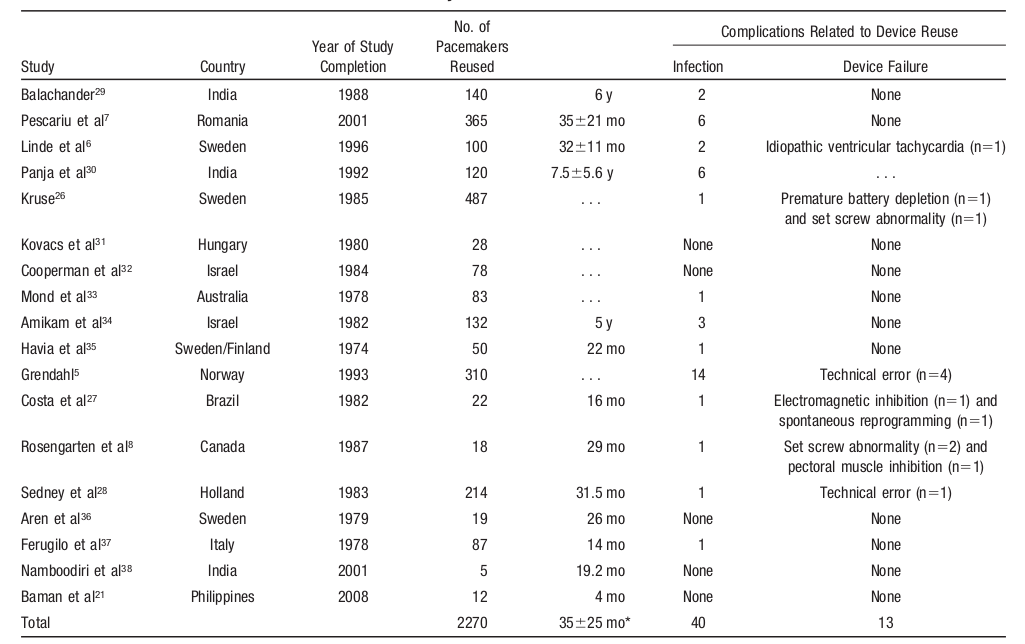
Baman TS, Meier P, Romero J, et al. Safety of pacemaker reuse: a meta-analysis with implications for underserved nations. Circ Arrhythm Electrophysiol. 2011;4:318–23.
Infections
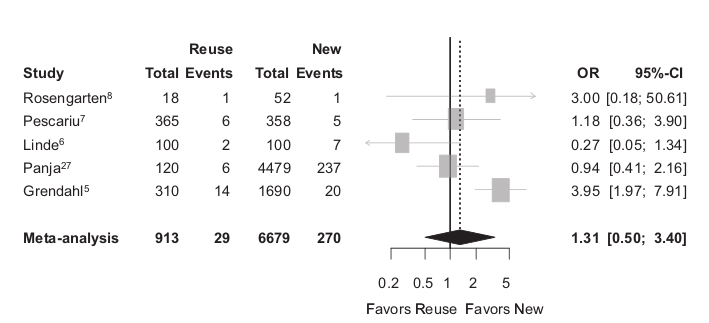
Baman TS, Meier P, Romero J, et al. Safety of pacemaker reuse: a meta-analysis with implications for underserved nations. Circ Arrhythm Electrophysiol. 2011;4:318–23.
Malfunction
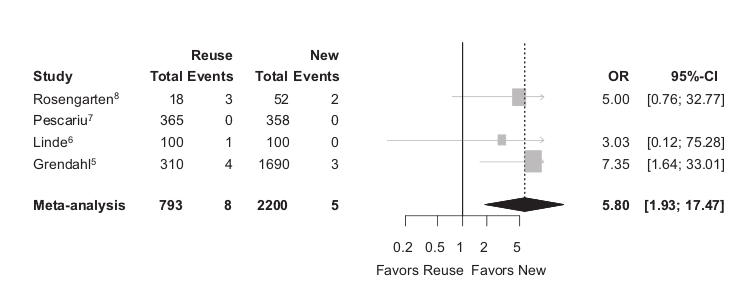
Baman TS, Meier P, Romero J, et al. Safety of pacemaker reuse: a meta-analysis with implications for underserved nations. Circ Arrhythm Electrophysiol. 2011;4:318–23.
JIPMER
Device reuse in JIPMER
- Stimdeveloppement (France)
- Explants at JIPMER
- Total devices ?
Protocol
- Clean - Enzymatic detergent / Wash / rinse
- Inspect - Discard if persistent blood stains
- Test - Battery status / function
- Pack / Label - Model / Manufacturer / Device / Sterilization date
- ETO sterilization
- Informed consent
- Follow up
Results
- 5 year period (2010 - 2015)
- 627 new devices and 260 reused devices (non randomized)
- Estimated longevity of reused device at implant - 10.5 yrs
- Malfunction - loss of capture due to loose set screw in 1, identified and corrected intra-op
- 6 month infection rate - 3/627 new vs 0/260 reused
- 3 deaths (ICD-2, CRT-1), unrelated to device
- Selvaraj RJ, Sakthivel R, Satheesh S, et al. Reuse of pacemakers, defibrillators and cardiac resynchronisation devices. Heart Asia 2017; 9: 59-62.
Ready for prime time?
Should be used widely
- Saves lives
- Saves money
- Reduces waste
What should be done to promote
- Document / follow up and publish results
- Standard protocols for reprocessing
- Third party reprocessors
- Government regulation
- Industry collaboration ?
CSI consensus document
- Nearly 1 million deaths annually due to lack of access to pacemakers
- Consent for device removal
- Properly cleaned, sterilized and reliably tested devices safe and effective
Kapoor A, Vora A, Nataraj G, Mishra S, Kerkar P, Manjunath CN. Guidance on reuse of cardio-vascular catheters and devices in India: A consensus document. Indian Heart J. 2017 May - Jun;69(3):357-363. doi: 10.1016/j.ihj.2017.04.003
Summary
Summary
- Currently reluctance on part of cardiologists and industry towards reuse
- Large body of evidence supporting reuse
- Standardized reprocessing at designated centers is needed
- Reuse of devices with adequate residual longevity should become standard